Use of Titration for Improving Quantitative Evaluation of Anti-HLA Antibodies by SAB
(P618) Use of titration for improving quantitative evaluation of Anti-HLA antibodies by SAB
Location: Platinum Ballroom
Poster Presenter(s)
Aim: Multiplexed flow cytometry-based single antigen bead (SAB) immunoassays are the current standard for detection and identification of anti-HLA alloantibodies. While highly sensitive and specific, well-recognized limitations of SAB including non-linearity, saturation, reagent variability, and potential for assay inhibition limit quantitative determination of alloantibody concentrations using the assay output of Mean Fluorescence Intensity (MFI). This limitation is a barrier to accurate assessment of potential clinical impact of anti-HLA antibodies. We hypothesized that anti-HLA antibody quantification by titration, as compared to MFI, will be a more informative measure of antibody quantity.
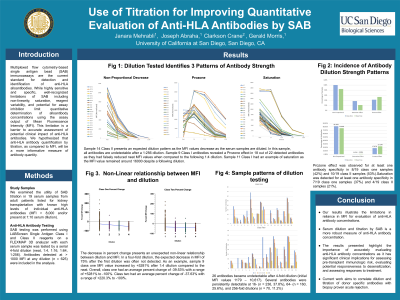
Method: We examined the utility of SAB titration in 19 serum samples from adult patients listed for kidney transplantation with known high levels of individual anti-HLA antibodies (MFI > 8,000 and/or present at 1:10 serum dilution). SAB testing was performed using LABScreen Single Antigen Class I and Class II reagents on a FLEXMAP 3D analyzer with each serum sample was tested by a serial 4-fold dilution (neat, 1:4, 1:16, 1:64, 1:256). Antibodies detected at > 1000 MFI at any dilution (n = 625) were included in the analysis.
Results: Serum dilution notably resulted in non-proportional decreases in MFI- e.g. the first 4-fold dilution resulted in an average MFI change of -7.2%, range -100% to +5,280% (excluding 2 cases of antigen excess prozone there were undetectable in neat serum). MFI increased after dilution in 135 antibodies. Conversely, 26 antibodies became undetectable after 4-fold dilution (initial MFI values 1179 – 10,617). Several antibodies were persistently detectable at 16- (n = 236, 37.8%), 64- (n = 160, 25.6%), and 256-fold dilutions (n = 70, 11.2%).
Conclusion: These results illustrate the limitations in reliance on MFI for evaluation of anti-HLA antibody concentrations. The results here indicate that serum dilution and titration by SAB is a more robust measure of anti-HLA antibody concentration. The ability to accurately evaluate anti-HLA antibody concentrations has significant clinical implications for assessing pre-transplant immunologic risk, evaluating potential responsiveness to desensitization, and assessing responses to treatment. Current work aims to correlate dilution and titration of donor specific antibodies with biopsy proven acute rejection.
Method: We examined the utility of SAB titration in 19 serum samples from adult patients listed for kidney transplantation with known high levels of individual anti-HLA antibodies (MFI > 8,000 and/or present at 1:10 serum dilution). SAB testing was performed using LABScreen Single Antigen Class I and Class II reagents on a FLEXMAP 3D analyzer with each serum sample was tested by a serial 4-fold dilution (neat, 1:4, 1:16, 1:64, 1:256). Antibodies detected at > 1000 MFI at any dilution (n = 625) were included in the analysis.
Results: Serum dilution notably resulted in non-proportional decreases in MFI- e.g. the first 4-fold dilution resulted in an average MFI change of -7.2%, range -100% to +5,280% (excluding 2 cases of antigen excess prozone there were undetectable in neat serum). MFI increased after dilution in 135 antibodies. Conversely, 26 antibodies became undetectable after 4-fold dilution (initial MFI values 1179 – 10,617). Several antibodies were persistently detectable at 16- (n = 236, 37.8%), 64- (n = 160, 25.6%), and 256-fold dilutions (n = 70, 11.2%).
Conclusion: These results illustrate the limitations in reliance on MFI for evaluation of anti-HLA antibody concentrations. The results here indicate that serum dilution and titration by SAB is a more robust measure of anti-HLA antibody concentration. The ability to accurately evaluate anti-HLA antibody concentrations has significant clinical implications for assessing pre-transplant immunologic risk, evaluating potential responsiveness to desensitization, and assessing responses to treatment. Current work aims to correlate dilution and titration of donor specific antibodies with biopsy proven acute rejection.

