Reduction of HLA Antibody in Pediatric Heart Patients After Daratumumab Treatment for Antibody Mediated Rejection
(P319) Reduction of HLA antibody in pediatric heart patients after daratumumab treatment for antibody mediated rejection
Location: Platinum Ballroom

Matthew Najor, PhD, F(ACHI)
University of Michigan Medicine
Poster Presenter(s)
Body: Anti-HLA antibody is a well understood and established to be a contraindication to transplant. Post-transplant complications such as with antibody-mediated rejection (AMR) due to donor specific anti-HLA antibodies (DSA) is a significant risk to decreased allograft survival in solid organ transplantation (txp). Current therapeutics, such as Rituximab, which fail to deplete B- and plasma- cells homed in the bone marrow. Current studies in kidney and heart have shown Daratumumab (Dara) to be a potential therapeutic in treatment of AMR. Dara is an IgG1κ anti-human CD38 monoclonal antibody and a first line therapeutic to target CD38+ B- and plasma-cells in treatment of patients with multiple myeloma. In this case series, we present the results of Dara treatment HLA DSA levels in pediatric heart post-transplant patients who failed to respond to our center’s standard protocol for AMR treatment.
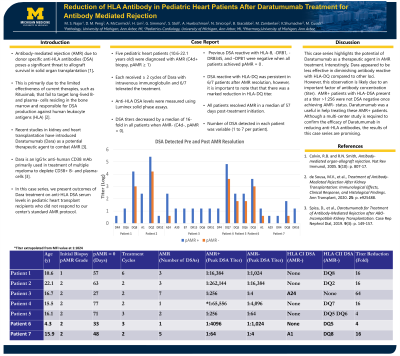
Seven pediatric heart patients (4.3 -22.1 years old) were diagnosed with biopsy proven AMR (C4d+, pAMR ≥ 1). Dara was combined with intravenous immunoglobulin (IVIG) and each patient received ≥ 2 cycles. Luminex solid phase assays was used to measure DSA levels and serial dilutions using a factor of 4 were performed on sera corresponding to AMR+ and AMR- biopsies. Titers for DSA decreased by a median of 16-fold in all patients when AMR- (C4d-, pAMR = 0). DSA reactive with HLA-DRB1, -DRB345, and -DPB1 were absent in patients when AMR-. Interestingly, HLA-DQ reactivity persisted in 6/7 patients when AMR-, however, there was a substantial decline in the corresponding DQ titers (Table and Figure). In addition to DSA reduction, an overall decrease in anti-HLA antibody profile was observed for all patients in a median of 3 months. AMR was resolved in a median of 57 days after treatment initiation of Dara. DSAs detected in each patient varied (1 to 7). This likely explains the disparity in titers varying between patients. Dara was well tolerated without significant side effects or documented infections in all patients.
Conclusion: Daratumumab has the potential for use as a therapeutic modality in treatment of AMR and as a valuable agent part of a desensitization program for highly sensitized patients needing a lifesaving transplant. The results of this case series are promising, however, a multi-center study approach is necessary to confirm the efficacy of daratumumab.
Seven pediatric heart patients (4.3 -22.1 years old) were diagnosed with biopsy proven AMR (C4d+, pAMR ≥ 1). Dara was combined with intravenous immunoglobulin (IVIG) and each patient received ≥ 2 cycles. Luminex solid phase assays was used to measure DSA levels and serial dilutions using a factor of 4 were performed on sera corresponding to AMR+ and AMR- biopsies. Titers for DSA decreased by a median of 16-fold in all patients when AMR- (C4d-, pAMR = 0). DSA reactive with HLA-DRB1, -DRB345, and -DPB1 were absent in patients when AMR-. Interestingly, HLA-DQ reactivity persisted in 6/7 patients when AMR-, however, there was a substantial decline in the corresponding DQ titers (Table and Figure). In addition to DSA reduction, an overall decrease in anti-HLA antibody profile was observed for all patients in a median of 3 months. AMR was resolved in a median of 57 days after treatment initiation of Dara. DSAs detected in each patient varied (1 to 7). This likely explains the disparity in titers varying between patients. Dara was well tolerated without significant side effects or documented infections in all patients.
Conclusion: Daratumumab has the potential for use as a therapeutic modality in treatment of AMR and as a valuable agent part of a desensitization program for highly sensitized patients needing a lifesaving transplant. The results of this case series are promising, however, a multi-center study approach is necessary to confirm the efficacy of daratumumab.
